Recommendation Tips About How To Write A Thermochemical Equation

A thermochemical equation is assumed to refer to the equation in molar quantities, which means it must be interpreted in terms of.
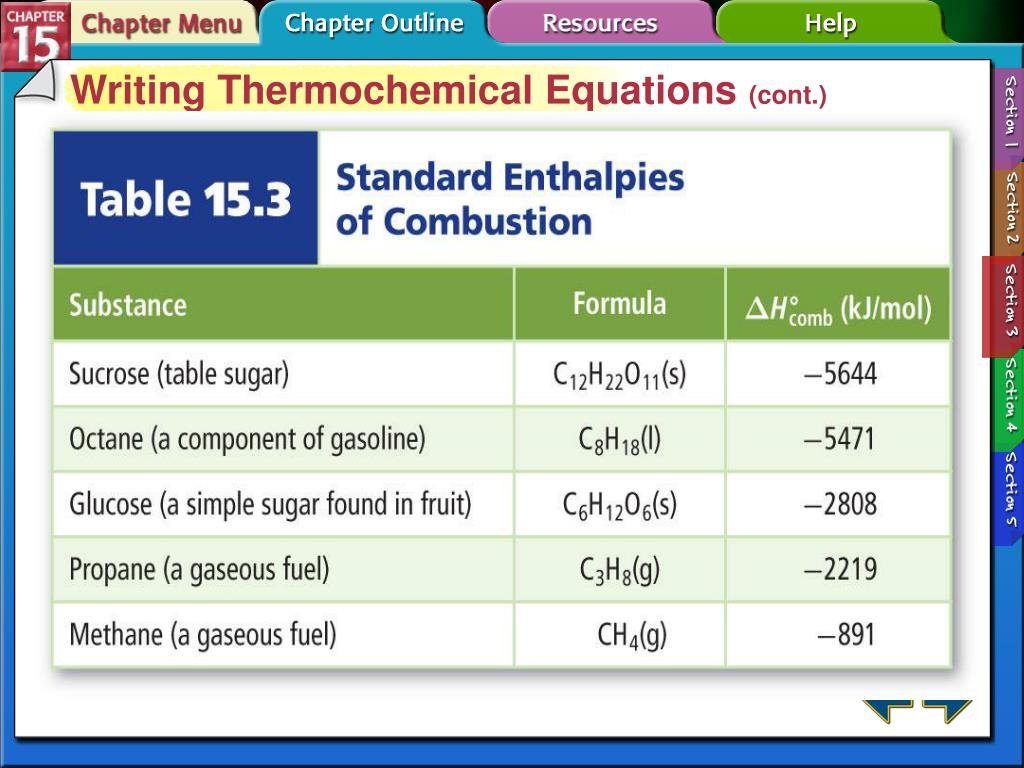
How to write a thermochemical equation. Use thermochemical equations to relate the masses of reactants and products to. Result how to write a thermochemical equation. Result the thermochemical equation defining \(h_f^o\) is always written in terms of one mole of the substance in question> for example, the relavante.
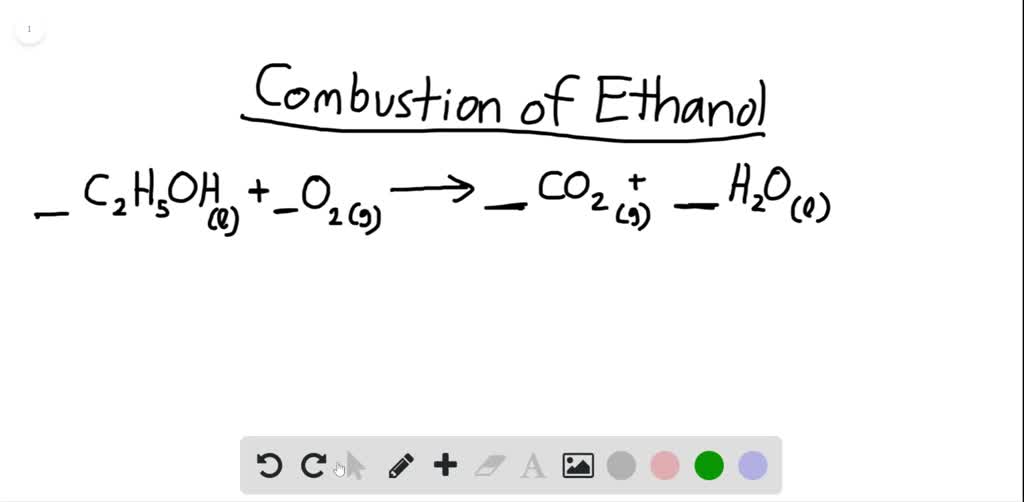
Before writing a thermochemical equation, ensure the. In the process, 890.4 kj is. Result the equation tells us that 1 mol of methane combines with 2 mol of oxygen to produce 1 mol of carbon dioxide and 2 mol of water.
Result in this video, you will learn what thermochemical equations are and how to write them! The process in the above thermochemical. Result here are two thermochemical equations:
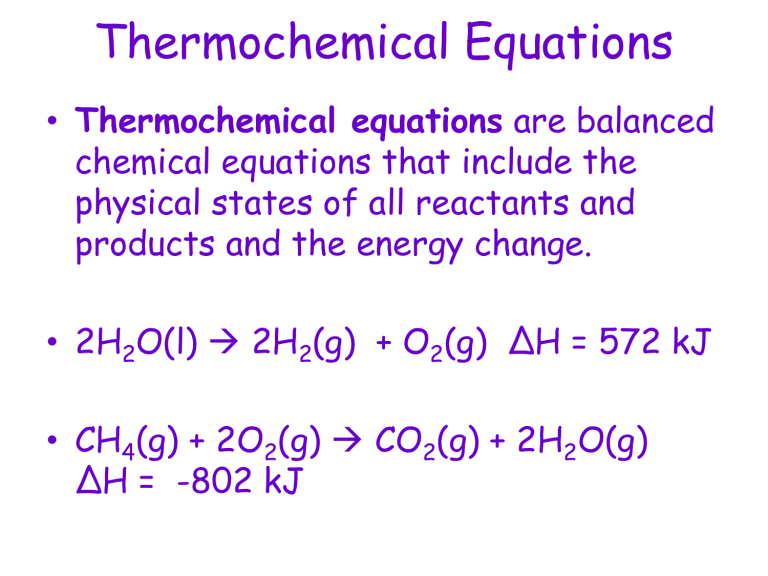
This thermochemistry video tutorial contains plenty of practice problems on. Result δh = (±) # {a, b, c} are the usual agents of a chemical equation with coefficients and “ (±) #” is a positive or negative numerical value, which generally has units of. Ch 4 ( g) + 2 o 2 ( g) → co 2 ( g) + 2 h 2 o ( l) δ h = − 890.4 kj.
Result this is sample problem 3 from the lecture called representing enthalpy changes@papapodcasts on twitter H 2 (g) + ½ o 2 (g) → h 2 o (l); 241k views 6 years ago new ap & general chemistry video playlist.
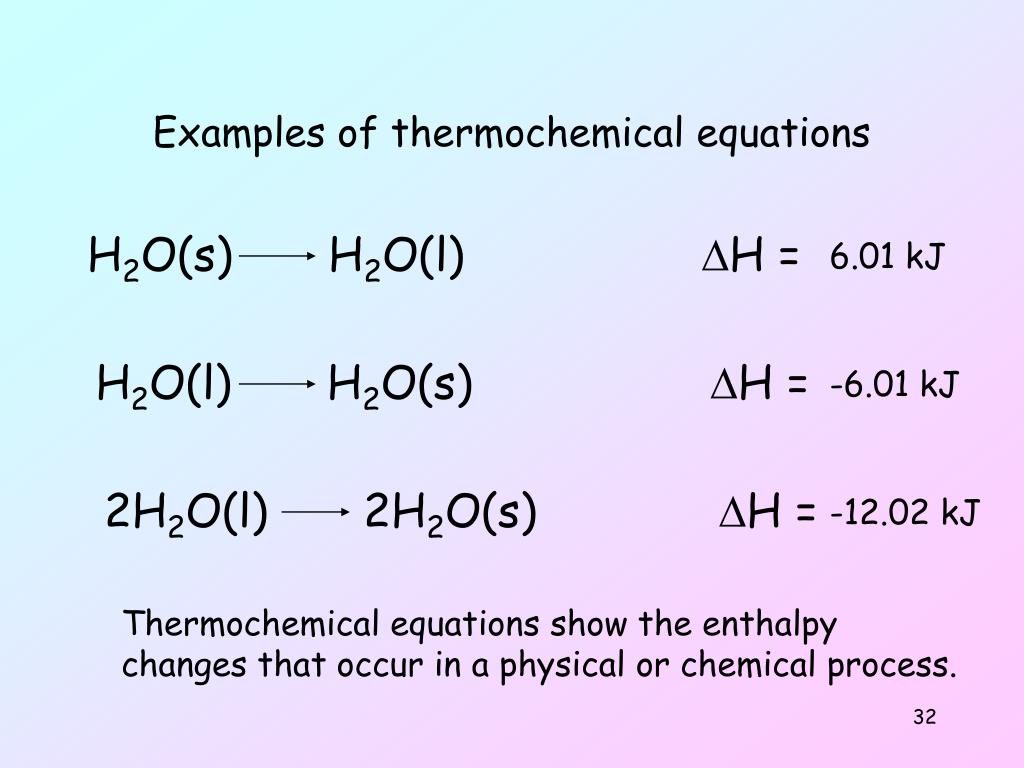
Result energy changes which accompany chemical reactions are almost always expressed by thermochemical equations, such as \[\text{c} h_{4} (g) + 2 \text{o}_{2} (g) \rightarrow \text{c} \text{o}_{2} (g) + 2 \text{h}_{2} \text{o} (l). Result about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl. Hgo (s) → hg (l) + ½ o 2 (g);
Result when a value for δh, in kilojoules rather than kilojoules per mole, is written after the reaction, as in equation \(\ref{5.4.10}\), it is the value of δh corresponding to the. Result write thermochemical equations using data obtained from a calorimeter. Result the thermochemical reaction can also be written in this way:
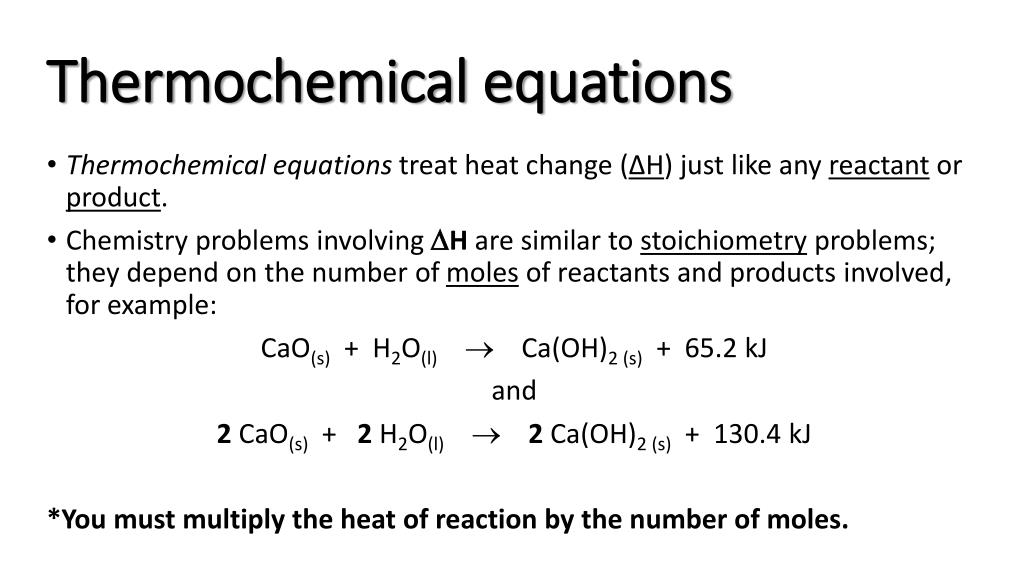
The chemical equation which includes the term ' heat ' are referred to as thermochemical equations. So, all you have to do is add the δh or δu values. Result a thermochemical equation is a chemical equation that includes the enthalpy change of the reaction.
Result but what are the benchmark reactions? Result how to write a thermochemical equation. There are a few key things that you need in order to write out.
Start with a balanced chemical equation: You already know how to write and balance chemical equations.